Applied Imaging Mass Spectrometry (AIMS) Core / Service Center
The Johns Hopkins Applied Imaging Mass Spectrometry (AIMS) Core / Service Center offers rapid matrix-assisted laser desorption/ionization (MALDI) imaging at high spatial resolution, which includes sample preparation, on-tissue digests and derivatizations, and data analysis. Spatially resolved MALDI imaging measurements are directly taken from a frozen or formalin-fixed paraffin-embedded (FFPE) tissue section without destroying it. MALDI imaging combines mass spectrometric analyses of biomolecules with simultaneous histological evaluation to analyze intact proteins, tryptic peptides (on-tissue tryptic digest), N-glycans (on-tissue PNGase digest), peptides, lipids, metabolites, drug molecules, and drug metabolites in a spatially resolved manner.
In 2022, we have added a second MALDI imaging instrument to the AIMS core through an NIH high-end instrumentation grant (S10 OD030500). This instrument is a timsTOF flex MALDI-2 instrument, which brings three new technologies to the AIMS core: (1) MALDI-2 ionization for significantly enhanced imaging sensitivity, (2) simultaneous trapped ion mobility spectrometry (tims) separation, (3) nano-LC-MS/MS capabilities.
In 2023, we will be adding a laser ablation (LA) inductively coupled plasma (ICP) time of flight (TOF) mass spectrometry imaging instrument to the AIMS Core through an NIH high-end instrumentation grant (S10 OD034239). This instrument will bring multiplexed elemental and metallomic tissue and cell imaging capabilities to the AIMS Core.
The Johns Hopkins AIMS Core is located in the Cancer Research Building 2 (CRBII) in the lower basement in rooms LB03E and LB08.
Project timelines for grant submissions: Please contact us 6 weeks in advance for assistance with grant submissions (without data collection), and 3 months in advance for collecting preliminary data for grant submissions.
For publications please acknowledge the AIMS Core as follows: We would like to acknowledge the instruments and expertise of the Johns Hopkins Applied Imaging Mass Spectrometry (AIMS) Core Facility at the Johns Hopkins University School of Medicine, which were critical in advancing this work.
For publications using the timsTOF fleX MALDI-2 instrument please acknowledge the NIH S10 grant it was purchased from: We would like to acknowledge NIH S10 OD030500 which funded the Bruker timsTOF fleX MALDI-2 instrument.
Major Equipment
Sample Preparation
The AIMS core facility is equipped with a variety of sample handling and automated sample preparation tools that allow the usage of controlled and reproducible protocols. Tissue samples are snap frozen at the site of collection or can be heat-treated to denature degradative enzymes. A Leica cryostat is available in the facility for cryo-sectioning of tissues at low temperatures without distorting molecular profiles. AIMS core staff closely collaborates with users to perform MALDI-imaging compatible cryo-sectioning in MALDI-compatible cryo-media in the facility. Protocols for MALDI imaging sample preparation of formalin-fixed, paraffin-embedded (FFPE) tissues are also well established, and are performed in the AIMS core facility.
Reproducible sample preparation is achieved by using the HTX M5 sprayer for accurate robotic spraying of enzymes for on-tissue digests, of derivatization agents for on-tissue derivatizations, and of matrices. Protocols for on-tissue digestions with trypsin and peptide N-glycosidase enzymes are available for proteomic and glycomic imaging investigations, respectively. Novel protocols for on-tissue derivatizations to detect amino acids and neurotransmitters are also available. AIMS core staff will perform all sample preparations as part of the MALDI imaging service provided. Customized development for novel sample preparation and MALDI imaging protocols is available in the AIMS core as well.
MALDI Imaging
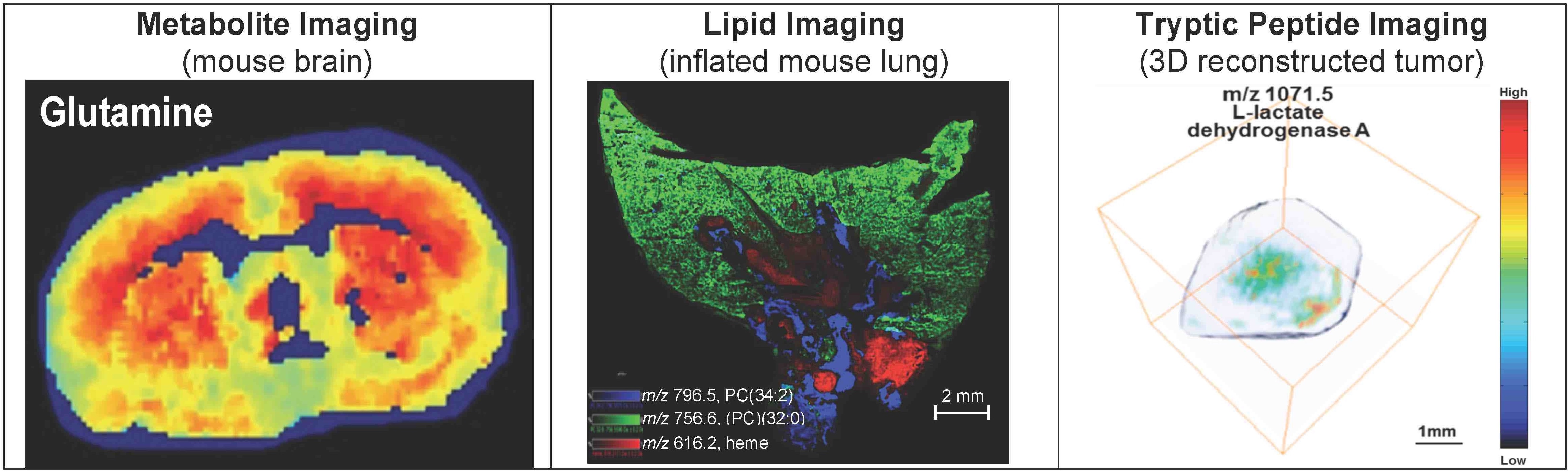
MALDI is currently the most commonly employed approach for imaging mass spectrometry (IMS) of biological samples as the soft energies involved enable ionization of intact biological molecules. The AIMS core facility has realized an infrastructure dedicated to highly multiplexed high-throughput MALDI imaging with a Bruker Rapiflex MALDI TOF/TOF instrument and a Bruker timsTOF fleX MALDI-2 instrument. The instruments are currently some of the fastest available instruments for IMS, capable of high-throughput multiplexed MALDI imaging of up to 5,000 biomolecules at once at a spatial resolution of 10-micron pixel size or better. MALDI imaging covers an area of a regular microscopy slide of 75 mm by 25 mm.
The AIMS core offers targeted MALDI imaging of drugs, drug metabolites, imaging agents, contrast agents, or other agents, which is developed in close collaboration with the user to set up customized MALDI imaging protocols. We also offer discovery MALDI imaging of metabolites, lipids, peptides, intact proteins, tryptic peptides, and glycans. Tandem mass spectrometry (MS/MS) is also available in imaging or profiling mode, using collision induced dissociation (CID) for confirming molecular identifications through fragmentation directly from tissue sections. The new timsTOF flex MALDI-2 instrument allows for MALDI-2 ionization for enhanced imaging sensitivity, simultaneous trapped ion mobility spectrometry (tims) separation combined with mass spectral separation, and nano-LC-MS/MS capabilities.
Following MALDI imaging, the MALDI matrix can be washed off, and histology or immunohistochemistry (IHC) staining can be performed on the same slide. Technical staff in the AIMS core performs standard histology and IHC protocols in close collaboration with users, followed by slide scanning for co-registered overlay with MALDI imaging data.
Data Analysis
MALDI imaging generates big data of, in some cases, up to hundreds of gigabytes per dataset. The AIMS core offers several data analysis solutions, including segmentation analysis, pathology-guided analysis, and statistical analysis with the dedicated MALDI imaging analysis software package SCiLS Lab Pro. Two workstations with the Bruker MALDI imaging and data analysis software packages FlexImaging, SCiLS Lab Pro, MetaboBASE, and Compass MetaboScape are available in the AIMS core for self-guided or assisted data analysis. These subscription software packages enable extended capabilities for statistical analysis of multiple MALDI imaging datasets of large size, as well as enhanced analyte identification.
Education
Not sure where to start with Mass Spectrometry imaging? Try our online course. Scan the QR code or go to https://bit.ly/JHMALDI
This course covers the basics of Mass Spectrometry Imaging and then focuses on MALDI imaging including sample preparation, on-tissue digests and derivatizations, and data analysis. It consists of eight modules of about one hour each with embedded quizzes, which, upon completion with a passing score, will award you a Johns Hopkins certificate.


Dr. Kristine Glunde, AIMS Core Director, aims@jhmi.edu
Dr. Caitlin Tressler, AIMS Core Assistant Director, aims@jhmi.edu
| Hours | Location |
|
Monday-Friday, 9am-5pm |
1550 Orleans Street Cancer Research Building II, Room LB03E and LB09 Baltimore, MD 21287 |
| Name | Role | Phone | Location | |
|---|---|---|---|---|
| Kristine Glunde, PhD |
Director
|
410-614-2705
|
aims@jhmi.edu
|
CRB II, Room LB 09 or Traylor Building, Room 203
|
| Caitlin Tressler, PhD |
Assistant Director
|
410-614-7959
|
aims@jhmi.edu
|
CRB II, Room LB 09 or Traylor Building, Room 309
|
| Dalton Brown, B.S. |
Research Specialist
|
dbrow273@jhmi.edu
|
CRB II, Room LB 09
|
|
| Cole Johnson, B.A. |
Research Specialist
|
njohn114@jhu.edu
|
CRB II, Room LB 09
|
|
| Gabbie Lagdameo, B.S. |
Lab Technologist
|
glagdam1@jh.edu
|
CRB II, Room LB 09
|